การตรวจวัดสีของเหลวทางเภสัชกรรม

สีของของเหลวทางเภสัชกรรมเป็นพารามิเตอร์การควบคุมคุณภาพที่สำคัญ การเปลี่ยนแปลงสีของของเหลวทางเภสัชกรรมอาจเป็นสัญญาณของการย่อยสลาย สิ่งเจือปน หรือการเปลี่ยนแปลงด้านประสิทธิภาพ การประเมินสีของของเหลวทางเภสัชกรรมเทียบกับมาตรฐานที่กำหนดไว้ล่วงหน้าเป็นกระบวนการควบคุมคุณภาพทั่วไปเพื่อให้แน่ใจว่าผลิตภัณฑ์ทางเภสัชกรรมมีคุณสมบัติตรงตามข้อกำหนดด้านคุณภาพและความปลอดภัย
มาตรฐานสียา
ที่เภสัชตำรับยุโรป (EP) และเภสัชตำรับของสหรัฐอเมริกา (USP)เป็นมาตรฐานสีที่กำหนดขึ้นซึ่งใช้กันอย่างแพร่หลายในอุตสาหกรรมยาเพื่อประเมินและสื่อสารสี สีและเฉดสีอ้างอิงของทั้งสีมาตรฐาน EP และ USP สร้างขึ้นจากการผสมและเจือจางโคบอลต์คลอไรด์ เฟอร์ริกคลอไรด์ และคอปเปอร์ซัลเฟต มาตรฐานสี EP ประกอบด้วยสีและเฉดสีอ้างอิง 37 สี ตั้งแต่สีน้ำตาล (B1 – B9) สีน้ำตาล/สีเหลือง (BY1 – BY7) สีเหลือง (Y1 – Y7) สีเขียว/สีเหลือง (GY1 – GY7) และสีแดง (R1 – R7) USP ประกอบด้วยสีและเฉดสีอ้างอิง 20 สีที่เรียกว่าจาก A ถึง T
วิธีประเมินสีของของเหลวทางเภสัชกรรม
มีวิธีการต่างๆ มากมายที่สามารถใช้ในการประเมินสีของเหลวทางเภสัชกรรม ได้แก่การประเมินด้วยสายตาและสเปกโตรโฟโตมิเตอร์– การประเมินด้วยสายตาเป็นวิธีการแบบดั้งเดิมและเกี่ยวข้องกับผู้สังเกตการณ์ที่เป็นมนุษย์ในการเปรียบเทียบตัวอย่างของเหลวทางเภสัชกรรมกับชุดมาตรฐานสี (เช่น EP, USP เป็นต้น) แม้ว่าวิธีนี้จะง่าย แต่ก็มีแนวโน้มที่จะเกิดอคติของมนุษย์ได้จากการรับรู้สีและข้อผิดพลาดนี้ สีและเฉดของสีของมาตรฐานในรูปของเหลวอาจเปลี่ยนไปตามกาลเวลา โดยต้องมีการเตรียมอย่างต่อเนื่องเพื่อรักษาความสามารถในการตรวจสอบย้อนกลับ
วิธีสเปกโตรโฟโตเมทรีเป็นวิธีการเชิงปริมาณที่เกี่ยวข้องกับการวัดการดูดกลืนแสงของตัวอย่างของเหลวทางเภสัชกรรมที่ความยาวคลื่นต่างกัน ข้อมูลนี้จะถูกประมวลผลเป็นค่าสีเช่นCIE L*A*B*ฯลฯ ซึ่งสามารถนำมาใช้ในการพัฒนาการเปรียบเทียบตามวัตถุประสงค์กับมาตรฐานที่ทราบ เช่น มาตรฐานสี EP และ USP วิธีการสเปกโตรโฟโตมิเตอร์ช่วยลดความจำเป็นในการประเมิน และความจำเป็นในการเตรียมและรักษามาตรฐานสีทางเภสัชกรรมเป็นประจำ
เครื่องสเปกโตรโฟโตมิเตอร์ CM-5 สำหรับการประเมินสีของเหลวทางเภสัชกรรม
โคนิก้า มินอลต้าสเปกโตรโฟโตมิเตอร์ CM-5เป็นเครื่องมือวัดสีแบบสแตนด์อโลนที่มีความอเนกประสงค์สูง ซึ่งสามารถจัดการและวัดสีในรูปแบบต่างๆ ได้อย่างง่ายดายตัวอย่างยา, จากผงและยาเม็ดเพื่อวางและของเหลว
CM-5 สามารถแสดงสีในพื้นที่สีและดัชนีต่างๆ เช่น CIE L*a*b*CIE L*C*H*, ดัชนีความขาว , ดัชนีความเหลือง เป็นต้น อีกทั้งยังมีดัชนี EP และ USP รวมถึงดัชนีมาตรฐานสีอุตสาหกรรมอื่นๆ เช่นระดับสีแพลตตินัม-โคบอลต์ (APHA/Hazen)–การ์ดเนอร์และเลขสีไอโอดีน ดัชนีที่ผู้ใช้กำหนดยังสามารถสร้างและนำไปใช้ได้เมื่อใช้ร่วมกับซอฟต์แวร์ข้อมูลสีSpectraMagic NX–
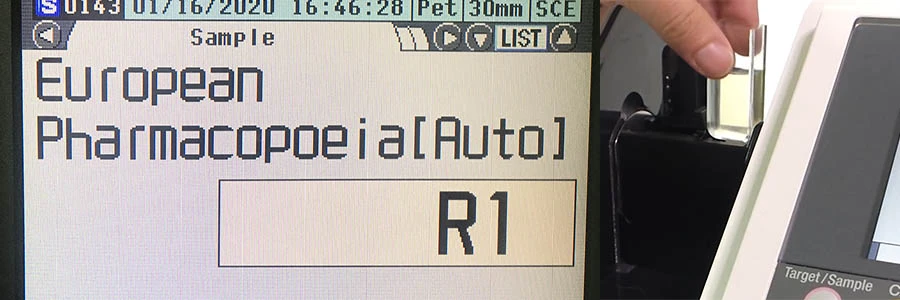
Spectrophotometer CM-5 วัดสีของสีของเหลวทางเภสัชกรรมโดยใช้ European Pharmacopoeia (EP)
การวัดสีของเหลวทางเภสัชกรรมทำได้ง่ายด้วย CM-5 วิดีโอนี้สามารถอธิบายเพื่อเรียนรู้เพิ่มเติมเกี่ยวกับความอเนกประสงค์และการทำงานที่เป็นมิตรต่อผู้ใช้ของ CM-5
หากต้องการความช่วยเหลือในการใช้งานระบบการวัดสีสำหรับการใช้งานสีทางเภสัชกรรม สามารถติดต่อกับผู้เชี่ยวชาญด้านสีของเราเพื่อรับคำปรึกษาฟรี
