Pharmaceutical Liquid Color Testing

The color of pharmaceutical liquids is an important quality control parameter. Any change in the color of pharmaceutical liquid can be a sign of degradation, impurities, or potency change. Evaluating the color of pharmaceutical liquids against pre-established standards is a common quality control process to ensure the pharmaceutical products meet the quality and safety specifications.
Pharmaceutical Color Standards
The European Pharmacopoeia (EP) and US Pharmacopeia (USP) are established color standards widely used within the pharmaceutical industry to evaluate and communicate color. The reference colors and shades of both the EP and USP color standards are created through mixing and diluting cobalt chloride, ferric chloride, and copper sulfate. The EP color standard consists of 37 reference colors and shades ranging from brown (B1 – B9), brown/yellow (BY1 – BY7), yellow (Y1 – Y7), green/yellow (GY1 – GY7), and red (R1 – R7). The USP consists of 20 reference colors and shades termed from A to T.
Ways to Evaluate the Color of Pharmaceutical Liquid
There are a number of different methods that can be used for evaluating pharmaceutical liquid color, including visual assessment and spectrophotometry. Visual assessment is the more traditional method and involves a human observer comparing the pharmaceutical liquid sample to a set of color standards (e.g., EP, USP, etc.). While this method is simple, it is prone to human bias in color perception and error. Furthermore, the color and shades of the standards, in liquid form, can drift over time, requiring continuous preparation in order to maintain their traceability.
The spectrophotometry method is a more quantitative approach that involves measuring the absorbance of light by the pharmaceutical liquid sample at different wavelengths. This information is then processed into color values like CIE L*a*b*, etc., which can further be used to develop an objective comparison with known standards like the EP and USP color standards. The spectrophotometer method eliminates subjectivity from the assessment and the necessity to prepare and maintain pharmaceutical color standards on a regular basis.
Spectrophotometer CM-5 for Pharmaceutical Liquid Color Evaluation
The Konica Minolta Spectrophotometer CM-5 is a highly versatile and stand-alone color measurement instrument that can easily handle and measure various forms of pharmaceutical samples, from powder and pills to paste and liquid.
The CM-5 is capable of expressing color in various color spaces and indices like CIE L*a*b*, CIE L*C*h*, whiteness index, yellowness index, etc. It also offers the EP and USP indices as well as other industrial color standard indices like Platinum-Cobalt Color Scale (APHA/Hazen), Gardner, and Iodine Color Number. User-defined indices can also be created and implemented when used together with color data software SpectraMagic NX.
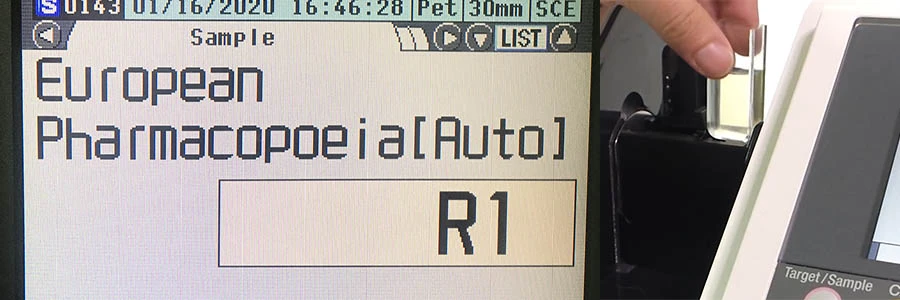
Spectrophotometer CM-5 measuring the color of pharmaceutical liquid color using European Pharmacopoeia (EP).
Pharmaceutical liquid color measurement is made simple with CM-5. Watch this video to learn more about the versatility and user-friendly operation of CM-5.
Need help implementing a color measurement system for your pharmaceutical color application? Get in touch with our color specialists now for a free consultation.
