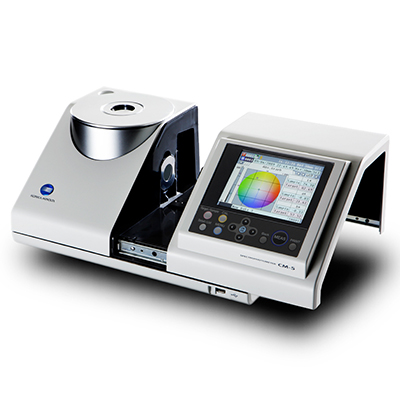
EP (European Pharmacopoeia) and USP (United States Pharmacopoeia) color standards are commonly used to evaluate liquid pharmaceuticals. Both EP and USP rely on visual assessment. This method has its limitation as it is prone to human error and is subjective.
Konica Minolta Spectrophotometer CM-5, equipped with both EP and USP indices, allows users to achieve accurate and repeatable color measurement of pharmaceuticals with ease. It also offers other industrial standard color indices and major colorimetric systems like APHA/Hazen, Gardner, Iodine Color Number, L*a*b*, and and L*C*h.
Need assistance with appearance, color, display, light measurement, hyperspectral imaging, or any other queries?
Please complete the fields below, and one of our authorized representatives will contact you promptly to discuss how we can assist.
All fields marked with an (*) are required.