Pharmaceutical Quality Inspections with Hyperspectral Imaging
Stringent regulations oversee the pharmaceutical industry, requiring the pharmaceutical manufacturing process to undergo numerous operational checks to ensure compliance with specific quality standards. While the traditional chemical-based method like high-performance liquid chromatography (HPLC) has been a cornerstone for pharmaceutical product quality determination, it is costly and time-consuming with limited throughput in high sample volume inspection. The demand for non-destructive and more efficient techniques has fueled the adoption of advanced technologies, and at the forefront is hyperspectral imaging (HSI).
Hyperspectral Imaging – An Overview
Hyperspectral imaging represents an advanced combination of imaging and spectroscopy techniques. Unlike traditional imaging methods that capture only the red, green, and blue color channels, hyperspectral imaging goes beyond, spanning a broad range of wavelengths across the visible and infrared regions. This unique capability allows hyperspectral imaging to capture a wealth of detailed spectral data for each pixel in an image, creating what is known as a hyperspectral cube. Within this three-dimensional dataset, each pixel possesses spatial coordinates along with an entire spectrum, offering a comprehensive and detailed view of the imaged scene or object.
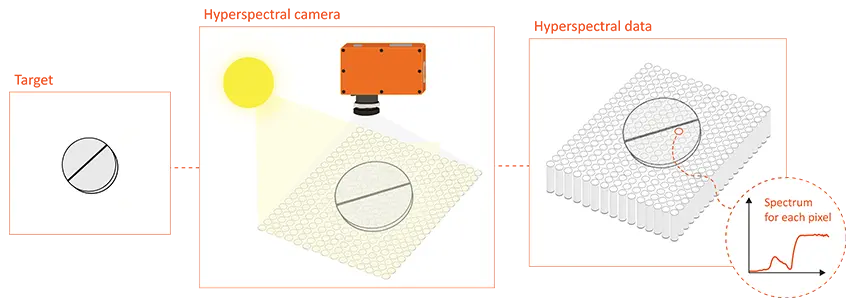
Illustrative example of hyperspectral imaging.
In hyperspectral imaging, the key lies in understanding the distinct spectral signatures of materials. Each material exhibits a unique pattern of light interaction (e.g., reflectance) at different wavelengths, forming a characteristic spectral fingerprint that can be utilized to distinguish the chemical or physical properties of different materials. With the potential for highly accurate and detailed material identification, hyperspectral imaging has emerged as a transformative technology in various industries such as agriculture, food, recycling, art conservation, and, notably, in pharmaceuticals for quality inspection.
How Hyperspectral Imaging Works in Pharmaceutical Quality Inspection?
Hyperspectral imaging, used interchangeably with chemical imaging, is well-documented in various pharmaceutical applications. Wilczynski et al. (2016) demonstrated the effectiveness of hyperspectral imaging in in discerning between genuine and counterfeit drugs. Meisner et al. (2023) effectively utilized hyperspectral imaging to assess the stability of uncoated tablets. Additionally, da Silva et al. (2019) showcased the efficacy of near-infrared (NIR) hyperspectral imaging for quantifying polymorphs in tablets with different multivariate models.
Hyperspectral imaging begins by capturing hyperspectral data through a specialized imaging system typically comprising a hyperspectral camera and a broad-spectrum illumination source spanning from visible to infrared wavelengths. The initial raw hyperspectral data then undergo pretreatment to eliminate noise and unwanted variations. Common pretreatment techniques in pharmaceutical settings include multiplicative scatter correction (MSC), standard normal variate (SNV), and Savitzky-Golay filtering. Subsequently, chemometric (multivariate) techniques are applied to extract valuable insights from the hyperspectral data for analysis, classification, or quantification. Some of the widely used chemometric techniques in pharmaceutical contexts include partial least squares (PLS) regression, Principal Component Analysis (PCA), and Multivariate Curve Resolution-Alternating Least Squares (MCR-ALS).
Specim Hyperspectral Imaging Cameras for Pharmaceutical Quality Inspection

Specim, a part of the Konica Minolta Group, is one of the leading providers of hyperspectral imaging solutions. Their lineup includes a variety of push-broom (line scan) hyperspectral cameras widely used across different industries. Particularly, the Specim FX series hyperspectral cameras found extensive applications in various industrial quality control and inspection, including the pharmaceutical sector. Specifically, the Specim FX 17 camera has been integral in hyperspectral imaging systems developed by reputable companies like SEA Vision and Indatech. These systems are instrumental in inspecting and verifying the quality of pharmaceutical products throughout the production process, from tablet manufacturing to final quality checks.
The Specim FX 17 hyperspectral camera presents a notable advantage due to its high spectral resolution and frame rate, which can cater to the precision and speed requirements of high-speed inspection of pharmaceutical products in the manufacturing or process development phase. Beyond its impressive capabilities, the compact Specim FX 17 hyperspectral camera comes with features that facilitate flexible and easy setup and implementation. Check out the video below to learn more about the Specim FX 17 hyperspectral camera.
WATCH VIDEO
For further information on Specim’s hyperspectral imaging cameras and solutions, or if you are considering integrating hyperspectral imaging into your pharmaceutical testing and inspection applications, feel free to reach out to us for a complimentary consultation.

